Technology and Markets
Introduction and IPSSL Technology and Markets
Lambdanetics develops and manufactures light emitter, detector, and processing products for the research, industrial, and military markets. The company currently focuses on IPSSL technology, a new and proprietary “atomic resonant” laser platform.
IPSSL technology is presented in three parts. Part 1 addresses performance features and market advantages. Selected applications are also briefly reviewed. In Parts 2 and 3, examples of resonant laser technology for environmental sensing are described. Specifically in Part 2, a UAV-compatible laser sensor for remote environmental moisture determination is discussed. In Part 3, a laser sensor for the selective and precise determination of calcium and strontium in seawater from a float platform is presented.
Part 1: IPSSL Technology and Applications Summary
Learn More
Part 2: A UAV-Compatible Laser Sensor for Remote Environmental Moisture Determination
Learn More
Part 3: A Laser Sensor for Selective and Precise Determination of Calcium and Strontium in Seawater from a Float Platform
Learn More
Part 1. IPSSL Technology and Applications Summary
INTRODUCTION
Lambdanetics develops and manufactures light emitter, detector, and processing products for the research, industrial, and military markets. The company currently focuses on IPSSL technology, a new and proprietary “atomic resonant” laser platform. IPSSL technology, performance features, and market advantages are presented. Selected applications are also reviewed.
OUTLINE
- Background
- Technological Features and Market Advantages
- Applications Summary
- Optical Pumps (DPAL)
- High Performance Chemical Sensing
- Environmental Chemical Sensing from Autonomous Platforms
BACKGROUND
IPSSL is a platform of atomic resonant lasers. Atomic resonant lasers are members of a class of emitters characterized by an output wavelength that is fixed precisely at a level corresponding to certain atomic transitions. A characteristic is that the output beam readily and efficiently interacts through optical excitation with specific atomic species having the matching atomic transition. Consequently, IPSSL technology is attractive for applications in:
- optical pumping,
- atomic clocks,
- spin exchange optical pumping,
- the generation of Bose Einstein condensates,
- and high performance, analytical chemical instrumentation.
In addition, the high accuracy and stability of the IPSSL wavelength are applicable to LIDAR and free space communications. Combined with its light weight and compact design, IPSSL is also especially attractive for chemical sensing from autonomous platforms, including unmanned aerial vehicles (UAV), and floats.
The term “atomic resonant” refers specifically to those atomic transitions terminating in ground states or metastable states. The IPSSL platform is anticipated to be broadly applicable to interactions with atoms with strong dipole-dipole transitions terminated in the ground states of metal vapors, including groups I (such as K, Rb, and Cs) and II (such as Ca, Sr, and Ba) elements and mercury, and terminated in metastable energy states, including those of rare noble (such as Ne, Ar, and Kr) gases. IPSSL platform models and emission wavelengths are given in Table 1. The model name includes the chemical symbol of the matching atomic species.
Table 1. IPSSL device models and wavelength of emissions.
| IPSSL Model | Wavelength (nm) |
| IPSSL-Hg | 404.657 |
| IPSSL-Hg | 546.075 |
| IPSSL-Ca | 422.673 |
| IPSSL-Ca | 657.278 |
| IPSSL-Sr | 460.733 |
| IPSSL-Ne | 633.443 |
| IPSSL-Ne | 640.225 |
| IPSSL-Li | 670.776 |
| IPSSL-Li | 670.791 |
| IPSSL-Ar | 706.722 |
| IPSSL-Ar | 714.704 |
| IPSSL-Rd | 780.027 |
| IPSSL-Rb | 794.76 |
| IPSSL-Kr | 785.423 |
| IPSSL-Kr | 810.437 |
| IPSSL-Xe | 823.1634 |
| IPSSL-Xe | 881.941 |
| IPSSL-He | 1083.034 |
The term (“atomic resonant”) is rooted in early work by Allan C.G. Mitchell and Mark W. Zemansky (” Resonance Radiation and Excited Atoms”, Cambridge University Press 1961) and others referenced therein, and the development of atomic resonance lamps. For example, the hollow cathode emitter (HCE) is an atomic resonant emitter used widely today in analytical instrumentation. HCEs generate atomic fluorescence of matched atomic species in flame spectrophotometry enabling precise atomic selection and quantitative analysis. Under similar conditions, an atomic resonant laser produces laser induced fluorescence (LIF). These same atomic transitions are the basis of other common, high precision, chemical analytical methods, including atomic absorption spectrophotometry (AAS) and inductively coupled plasma atomic emission spectroscopy (ICP-AES).
Until the recent introduction of the IPSSL platform to the market, tunable semiconductor lasers with stabilized wavelengths have been the only available atomic resonant device. DPAL lasers are also of the resonant class, but are not commercially available to our knowledge. Although some dye lasers fall into the category, their use has been supplanted by semiconductor devices.
TECHNOLOGICAL FEATURES and MARKET ADVANTAGES
Several technical features of IPSSL technology provide important market advantages compared to conventional, stabilized, tunable semiconductor lasers. These features are: (1) intrinsic locking of the wavelength; (2) close overlay of line profiles of IPSSL emission and absorption of matching atomic species; (3) temperature-independent wavelength.
1. Intrinsic Wavelength Locking
A key feature of IPSSL technology is that the peak wavelength (wavelength centroid) is intrinsically locked without the use of either mechanical servos or reference cells. Intrinsic locking is accomplished through the innovative Embedded Atomic Standard (EAS), confidential and proprietary (patent pending) technology of Lambdanetics. All commercial, stabilized semiconductor lasers require mechanical servos or reference cells (gas or metal vapor) to achieve locking. These lasers include Distributed Bragg Reflector lasers (DBR), Distributed Fiber Bragg lasers (DFB), Vertical Cavity Surface Emitting Lasers (VCSEL), Extended Cavity Tunable Lasers (ECLD), and Extended Cavity Tapered Lasers. Servos include holographic or other micro-gratings, 3-dimensional VBGs (volume Bragg grating), or sophisticated mechanical drives for diffraction gratings. As a result, the integration of IPSSL technology in sensors and other instrumentation promotes design simplicity, lower hardware cost, and greater compactness.
Furthermore, EAS operates continuously in real time. Consequently, IPSSL devices never go out of calibration: accuracy and stability (over time) are very high. An invariant wavelength is highly advantageous for applications in chemical instrumentation. In contrast, semiconductor lasers often require frequent and periodic calibration to minimize errors in the chemometric algorithms. Eliminating this variable promotes design simplicity and reduced hardware, operating, and maintenance costs.

2. Close Overlay of IPSSL Emission and Matching Atomic Absorption
Not only does EAS assure locking of the wavelength, but the line shape of an IPSSL emission closely overlays the absorption spectrum of the matching atomic species, as well. A relevant characterization parameter is the spectral full width at half maximum (FWHM). As an illustration, the overlay of the emission profile of an IPSSL-Rb device is compared to the absorption curve of rubidium vapor at various temperatures in Figure 1.
A consequence of this close overlay is efficient energy transfer of an optical absorption process involving an IPSSL and the matching element. This is important for optical pump applications, as discussed in more detail in the Applications Section below.

3. Temperature-Independent Wavelength
The wavelength of an IPSSL laser has been found to be independent of temperature over a relatively wide temperature range of up to about 5 degrees C due to EAS. By comparison, the wavelength of a typical, high quality semiconductor laser varies by about 7 pico-meters per degree C requiring sophisticated temperature control for many applications. This feature of IPSSL technology also promotes design simplicity and reduced hardware cost.

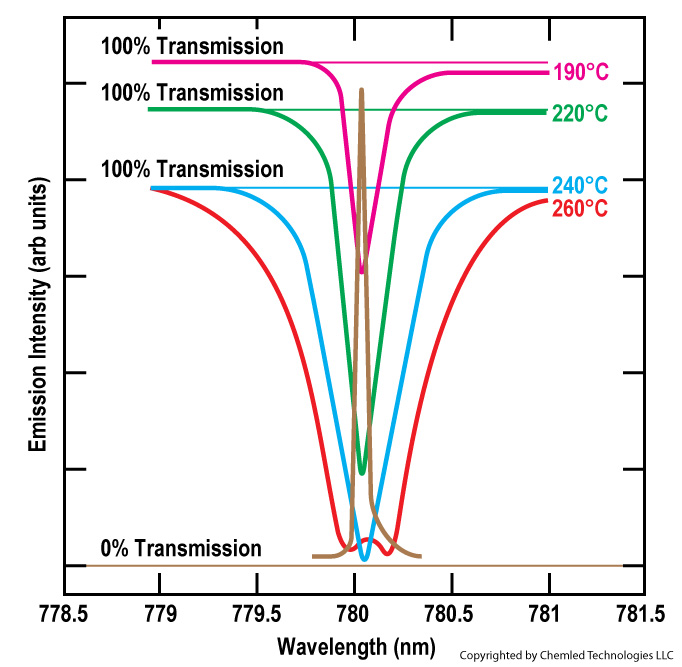
Figure 1-1. Overlap of IPSSL-Rb emission profile (brown) and absorption curves of rubidium. To generate absorption curves, a cell containing pure rubidium vapor with an optical path of 1 millimeter was placed in a collimated beam propagating from a QTH broadband lamp. Absorption curves were recorded using an FTIR with resolution of 1 cm-1. Excellent overlap of the IPSSL emission with the rubidium absorption is seen over the temperature range of from 190 to 260o C. This temperature range is important for applications in DPAL lasers and atomic cloc
APPLICATIONS
The features of IPSSL technology open the door to applications in optical pumping (DPAL in particular), high performance chemical sensing, chemical sensing from autonomous platforms, LIDAR, and atomic clocks, among others. Selected applications are discussed below.
1. DPAL Optical Pumps
The operation of diode-pumped lasers (DPL) is based on the transfer of energy through optical absorption from a laser diode pump to the gain medium of the DPL. A DPAL (diode-pumped alkali laser) contains an alkali metal gain medium in the vapor state. Alkali metal atoms in their ground state initially absorb the pump energy and enter an excited state. Atoms in the excited state undergo an internal energy transition between atomic levels. Energy is emitted at a higher wavelength (lower energy) than that absorbed from the pump in a stimulated emission process. Matching of the pump wavelength to the DPL absorption transition is critical to overall system efficiency. IPSSL lasers are inherently more efficient than semiconductor lasers for this application because of the close overlay of the absorption curve of the gain medium and the emission of a matched IPSSL, as demonstrated in Figure 1.
Semiconductor lasers are currently the device of choice for high power pump applications due to continued development of cooled semiconductor array technology. However, semiconductor lasers do not benefit from EAS. The wavelength is controlled by well known laser design parameters, including the composition and structure of epitaxial materials, device and packaging architecture, and temperature. Once the desired wavelength is reached, options are generally quite limited for modifying the FWHM. This is especially true for high power applications where the laser line is commonly already too broad, and the laser line width tends to increase as the power increases. The use of servos, such as volume Bragg gratings (VBGs), has partially alleviated the problem, but introduces additional temperature sensitivity to the pump.
To overcome this inflexibility of semiconductor lasers for DPAL pump applications, the gain medium of the DPAL can be operated at high pressure. This approach takes advantage of collisional broadening of the absorption profile of the gain medium to achieve better matching to the pump. However, high pressure design and operation adds enormous complexity and cost to a DPAL system.
The use of IPSSL pumps eliminates the need for both high pressure operation of the DPAL and the utilization of a servo in the pump.
2. High Performance Chemical Sensing
The wavelength accuracy and stability of IPSSL technology makes possible highly selective and precise chemical analytical instrumentation, such as enhanced emission and absorption spectrophotometry. There are several reasons for this. First, the “perpetual” calibration feature provides and invariant wavelength supportive of high precision. Second, IPSSL is the laser of choice when the detection of a matching element is to be analyzed. For example, IPSSL-Ca is selected for the determination of calcium in seawater (discussed in more detail Part 3). Finally, these sensors can support multivariate detection and signal processing for enhanced precision and sophistication of chemometric analytical models.
3. Chemical Sensing from Autonomous Platforms
The compactness and light weight of IPSSL technology combined with its high wavelength accuracy and stability is especially attractive for applications in environmental monitoring from autonomous platforms such as drones or floats. NIR and SWIR hyper-spectral imagers are being developed for similar applications. The comparative advantage of an IPSSL-based sensor is that the optical output power of the emitter is at least 10 times lower than that required by a conventional system. Consequently, IPSSL can be operated over the full range of sun intensities with no susceptibility to background interference caused by cloud cover, geographic variations, or surface conditions. The power budget of the system is also reduced promoting lower cost and longer battery life and mission up time.
IPSSL-based sensors offer additional advantages. First, beam quality can support spatial resolution readily exceeding anticipated requirements. In addition, operating wavelengths can be compatible with low cost, high performance, silicon-based detectors and CCDs needed in the detection channel.
Part 2. RESONANT LASER TECHNOLOGY FOR ENVIRONMENTAL SENSING
A UAV-Compatible Laser Sensor for Remote Environmental Moisture Determination
INTRODUCTION
Lambdanetics develops and manufactures light emitter, detector, and processing products for the research, industrial, and military markets. The company currently focuses on developing IPSSL technology, a new and proprietary “atomic resonant” laser. IPSSL offers high performance in a compact package enabling laboratory-quality chemical analytical measurements in an autonomous platform. Applications include a remote laser sensor (RLS) for the determination of the moisture content of soil or vegetation from a UAV-compatible platform. RLS is presented and discussed.
OUTLINE
- Drought-Driven Need for Moisture Sensor for Agricultural Water Management and Environmental Monitoring
- Commercial Opportunity and Socio-Economic Benefit
- The Innovation: An Affordable, UAV-Compatible Moisture Sensor
- Product Development Plan
NEED FOR MOISTURE SENSOR FOR AGRICULTURAL WATER MANAGEMENT AND ENVIRONMENTAL MONITORING
Federal, state, and local governments and the agricultural business sector face major challenges in managing drought-related economic and environmental conditions afflicting the State of California and other western regions of the United States. Drought conditions are straining regional socio-economics and the possibility that the drought could last a decade or longer is a real threat. To address this threat, sound scientific data, analysis, and modeling of relevant systems are needed to support the development of effective public policy, and to improve agricultural management practices.
In drought-prone regions where farming is irrigated, moisture content of soil and vegetation are key parameters in guiding water resource management. Given sufficient and timely data, the amount and frequency of water application could be controlled at the point of use to maximize overall water usage and crop yield. Available systems for determining soil moisture content are limited in several respects. Satellite radar systems [1] are designed to gather information on a global scale, and the typical resolution of about 40 kilometers is unable to distinguish the average farm in California. The depth of penetration of radar is also limited to a few centimeters. Gravimetric (direct) methods involve the collection of samples from the field and transporting them to a laboratory for analysis. While accurate, they are labor intensive. In-situ (indirect) sensors measure a property of the soil, such as dielectric constant, which can be correlated to the moisture content. These sensors [2-15] are fixed in position underground during operation, and their application is generally limited by labor intensity, hardware cost, safety concerns, and measurement grid resolution.
An improved sensor is needed combining several features. Operation needs to be reliable in full daylight, and insensitive to variations in cloud cover. Also, adequate spatial resolution is needed to enable mapping and graphical analysis. Such capability would enable the overlay of important data, such as crop yield and history of soil moisture content. In addition, the technique needs to be non-destructive. Furthermore, the cost needs to be affordable to support data acquisition on a weekly basis.
COMMERCIAL OPPORTUNITY AND SOCIO-ECONOMIC BENEFIT
RLS would provide the agricultural business community and government with an effective and affordable means of quantitatively monitoring drought conditions. In doing so, the broader socio-economic impact of drought could be ameliorated. Better tools to improve agricultural practices would also become available.
According to available documentation in the State of California (California Department of Water Resources, Public Policy Institute of California, University of California Cooperative Extension, LA Division of Agriculture and Natural Resources, and numerous magazine and newspaper articles), there are currently about 58,000 growers producing about $25B in agricultural goods, including 250 crops combined of vegetables, fruits, nuts, and flowers. About 9.6 M acres are irrigated consuming about 34 M acre-feet of water annually, or about 80% of the water delivered from below ground reservoirs and the diversion of ground water. In 2015 the U.S. Interior Department Bureau of Reclamation reduced the total water allocation to the irrigation network in the central region of California by 20%, and the State of California also imposed a state-wide reduction of 25%. As a result, the cost of an acre-foot of water for agricultural use has skyrocketed from about $140 several years ago to a recent price of over $2000 in some areas in the southern portion of the state. In turn, depending on the region, it is actually becoming more profitable to withhold planting, and to sell water allocations to other needy farms.
In the State of California a number of water conservation methods are being used. These include irrigation scheduling based on soil-plant or atmospheric measurements to determine when to irrigate. Using a more scientific approach has generally shown to decrease water usage while improving crop yield. Tail-water return systems aim to reuse water runoff. In addition, improvements in irrigation systems have been beneficial. This has included a shift away from surface irrigation toward pressurized irrigation (sprinklers, drip, micro-irrigation). The use of canal liners and improved canal structures has also been shown to reduce waste. Remote monitoring and water control systems also allow water districts to improve water management.
Demand for both water-saving hardware and services is created by prolonged drought conditions. The market value (total available market or TAM) corresponds to the financial savings brought about through the use of such hardware and services. These savings can be estimated to be equal to the potential financial loss due to the lack of water. A 20% cut in water supply could result in at least a loss of about 10% of crop production, or about $2.5B statewide. Assuming that a farmer would spend fifty cents to save one dollar of production (a 50% annual return on investment), the estimated TAM would be at least $1B annually. Viewing sensor hardware and sensing services as an integral segment of a technically advanced conservation market, the value of this segment in California alone is estimated to be at least 10% of the TAM, or about $100M annually. Combined with national and international markets, the worldwide TAM would be much larger.
THE INNOVATION: AN AFFORDABLE, UAV-COMPATIBLE MOISTURE SENSOR
The Remote Laser Sensor (RLS) meets the above-mentioned needs. RLS is an active spectral probe containing its own light source and uniquely configured detection system. Measurements are therefore insensitive to background noise [16,17] including variations of light intensity (cloud cover), geography, and surface conditions. RLS also provides full daylight operation without the sensitivity to high background noise encountered by other non-resonant techniques. These are major advantages compared to conventional mapping techniques, including NIR and SWIR hyper-spectral [16-25] imaging
In addition, beam quality of RLS would support spatial resolution as low as a few millimeters readily exceeding anticipated requirements.
Furthermore, operating wavelengths are compatible with the third overtone of water absorption [26-30], an ideal range for low cost, high performance, silicon-based detectors and CCDs needed in the detection channel of the RLS.
Finally, RLS can support multivariate detection and signal processing. For example, multiple wavelengths can be used supporting high precision and advanced chemical selectivity. In addition, the detection and analysis of non-optical variables such as temperature can support more sophisticated analytical models.
The technological foundation of the RLS is the IPSSL platform. A dual-wavelength enhancement would provide the most compact and affordable sensor.
IPSSL Technology is Foundation of RLS
IPSSL is an atomic resonant (see Part 1 of the Technology and Markets Section of this website) laser. The wavelength (centroid) of the spectral line is fixed at an atomic transition by virtue of EAS (embedded atomic standard) assuring continual absolute accuracy and perpetual calibration. No external servo (VBG, DBR, or other opto-mechanical device) is contained. Wavelength accuracy is specified at + 1 pico-meter. The wavelength is also very stable over time with a typical specification of 0.5 pico-meters over 12 hours. Available power matches commercial semiconductor devices. IPSSL is compact in design in view of its relative stability and power performance. For example, the IPSSL-Rd (see Product Data Sheet in this website) weighs about 2.4 lbs and has dimensions of 2”x3”x6”. Beam divergence is only 2-3 milli-radians.
An enhancement of IPSSL performance under developed to support RLS is dual-wavelength capability. This feature provides a differential signal improving selectivity and precision, as discussed in more detail below.
Operating Principles of IPSSL-Based RLS
The basic operation of and RLS sensor is depicted in Figure 1. A multi-wavelength beam produced by a dual wavelength IPSSL exits the autonomous platform. The two wavelengths are 780 (Rb) nm and 852 (Cs) nm. These wavelengths correspond to different regions of the known water absorption band. The utilization of multiple wavelengths provides a differential signal proportional to the moisture content of the soil improving the quality of the analytical measurement. As the exit beam strikes the sampled area, a portion of the light is both specularly and diffusely reflected from the surface, and both undergo absorption by water at the surface. The diffused light also penetrates deeper into the sampled area. As this penetration occurs, the light simultaneously continues to be absorbed and back-reflected toward the surface from various depths. The penetration depth at these wavelengths could be expected up to approximately 5 centimeters for dry vegetation or 1-2 cm for soil depending on conditions [19], [27], and [28]. Light received at the detector is a mixture of specularly reflected and scattered light that has interacted with water in the soil.
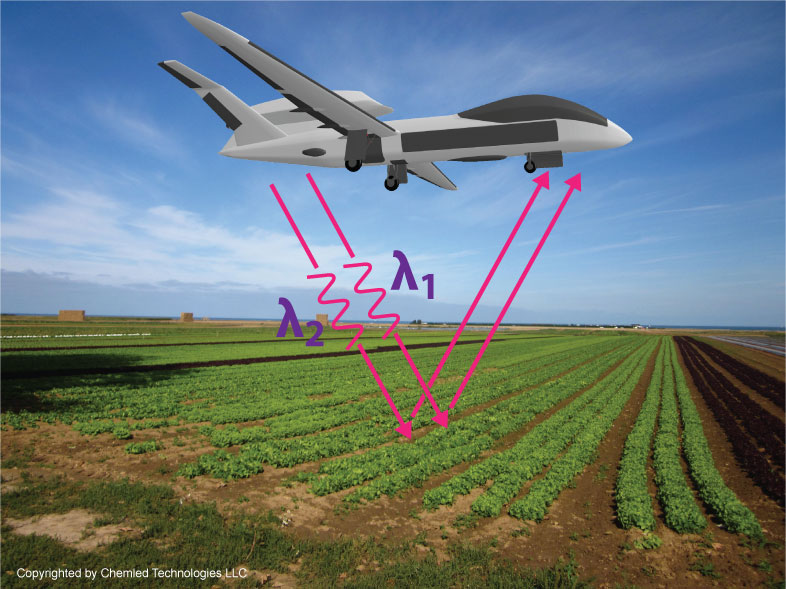
Figure 2-1. Depiction of RLS sensor in drone-based platform.
Unique features of the RLS design enable operation at low optical powers while maintaining reliability in full daylight. To illustrate, a 5 milli-watt laser pointer can barely be seen by eye in full daylight. However, a similar resonant light source (i.e., IPSSL) configured in the RLS could easily be detected several hundred feet away. Conventional (non-resonant) semiconductor lasers would have to be operated at much higher power. Resulting compactness, light weight, and low power consumption are compatible with the UAV platforms. Cost is also relatively low.
A final benefit of using low power is compliance with FDA CDRH IIIA regulations.
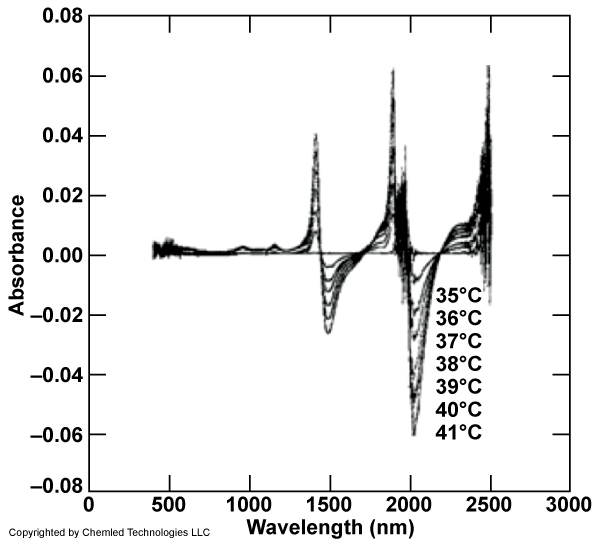
Figure 2-2. Dependence of water absorption on temperature in the NIR spectral range of from 500-2300nm, which in an important input to data analysis. Each presented plot is the residual band shape after subtracting the reference spectrum of water at 35 deg C. NIR absorbance spectra were taken at 35-41 deg C using 1mm cuvette. The sensitivity to the third overtone of water is on the order of milli-absorbance unit / deg C. Unpublished data – D. Sobczynski.
The RLS sensor would ideally contain a radio-metrical temperature sensor allowing the absorption coefficient of water to be corrected (see Figure 2) for temperature. The empirical multi-variate data would be processed by 2-D chemometrics.
PRODUCT DEVELOPMENT PLAN
Lambdanetics plans to enter the market for both equipment sales and leasing. A 3-stage roadmap for product and market development is being pursued.
Demonstrating the feasibility of the dual-wavelength IPSSL with stable characteristics and narrow linewidth is the first stage.
The second stage is the development of a drone-ready RLS engineering prototype. Lambdanetics has plans to team with a drone manufacturer to execute this task. Critical features for drone compatibility are being identified to guide the design of the prototype. One such feature is the utilization of a stationary (i.e., non-scanning) light source to promote light weight and compactness. Also, an integrated, light weight, CCD camera would provide registration (i.e., precise overlay) of maps of moisture content to actual geographic position. The outcome of this phase would be a functional, UAV-compatible sensor with connectivity to a data processing module.
The UAV business is its infant stage. Drones have actually been used for photography and video production for a number of years, which currently constitute the largest market. Several companies are already involved, including Aerial MOB, Helivideo Produtions, Pictorvision, RC Productions, and SnapVideo. The FAA initiated an approval process for line-of-sight (less than 500 feet) operation in 2014, which may tend to slow growth. GIS (geographic information systems, such as surveying and mapping) is the second largest market for drones due to the high resolution of imagery and short turn-around time. Companies that offer drones and drone services include Phoenix Aerial (www.phoenix-arial.com), Xact Sense (www.xactsense.com), Coptercraft (www.coptercraft.com), and Precision Hawk (www.precisonhawk.com). Phoenix Arial is currently offering the Riegl (www.riegl.com) fiber optics gyro sensor in their Ranger System, which is used for survey-grade mapping, and the Velodyne LIDAR system (www.velodynelidar.com) in their Scout System, which is also used for mapping. MicraSense has also recently announced intensions to offer photographic services for agricultural use. Investment in this field is growing, and timing for developing the right partnerships is excellent.
The third stage is “application methodology”. This effort requires close collaboration with experts in several fields, including hydrologists, agriculturalists, and land managers, to develop a methodology for the practical application of RLS data. The methodology will involve the complimentary use of established active or passive methods of moisture content measurement for validation purposes.
Finally, regarding risks to commercialization, it is believed that the greatest risk will be market adaptation for the following reason. Based purely on third order absorption coefficients of water [26-30], the penetration depth of the light from the RLS (in pure water) could be several centimeters. Diffuse reflectance (scattering) of the light within soil reduces this working depth. Consequently, RLS data would presumably be directly relevant to the critical germination stage of a crop when the root system is shallow. More sophisticated models would be required for mature crops to correlate RLS data through soil physics to moisture-depth profiles. Because the embedded atomic standard of IPSSL is time invariant, data acquired by RLS will be universally comparable by different laboratories. In addition, RLS mapping data could be correlated to conventional, stationary sensors to extract moisture depth profiles.
It is important to emphasize that RLS analysis is not limited to the soil alone. The direct measurement of the moisture content of vegetation, fruit, and grains is also possible. In fact, water moisture is routinely determined in grain processing by using various NIR absorption and reflectance instruments. Furthermore, USDA utilizes NIR spectroscopy as an approved technique for the determination [31 and references therein] of moisture in grains.
REFERENCES
1. http://aquarius.umaine.edu/cgi/search.htm
2. N.Rodriguez-Alvarez et al., Review of crop growth and soil moisture monitoring from a ground-based instrument implementing the Interference Pattern GNSS-R Technique, Radio Science, vol 46 issue 6, 2011
3. Albergel, C., De Rosnay, P., Gruhier, C., Munoz-Sabater, J., Hasenhauer, S., Isaksen, L., Kerr, Y., Wagner, W., 2012: "Evaluation of remotely sensed and modeled soil moisture products using global ground-based in situ observations." Remote Sensing of Environment 118(0): pp. 215-226
4. Anderson, M.C., Norman, J.M., Mecikalski, J.R., Otkin, J.A., & Kustas, W.P. (2007). A climatological study of evapotranspiration and moisture stress across the continental United States based on thermal remote sensing: 1.Model formulation. Journal of Geophysical Research: Atmospheres, 112, D10117
5. Brunini, O. and G.W. Thurtell, 1982: An improved thermocouple hygrometer for in situ measurements of soil water
potential. Soil Science Society of America Journal, 46, pp. 900–904.
6. Campbell, D.J. and J.K. Henshall, 2001: Bulk density. In: K.A. Smith and C.E. Mullins, Soil and Environmental Analysis:
Physical Methods, Marcel Dekker, New York, pp. 315–348
7. Dirksen, C., 1999: Soil Physics Measurements. Catena Verlag, Reiskirchen, Germany, 154 pp.
8. Dirksen, C. and S. Dasberg, 1993: Improved calibration of time domain reflectometry soil water content measurements.
Soil Science Society of America Journal, 57, pp. 660–667.
9. Gardner, W.H. and C. Calissendorff, 1967: Gamma- ray and neutron attenuation measurement of soil bulk density and water content. Proceedings of the Symposium on the Use of Isotope and Raditation Techniques in Soil Physics and Irrigation Studies (Istanbul, 12-16 June 1967). International Atomic Energy Agency, Vienna, pp. 101–112.
10. Gardner, C.M.K., D.A. Robinson, K. Blyth and J.D. Cooper, 2001: Soil water content. In: K.A. Smith, and C.E. Mullins, Soil and Environmental Analysis: Physical Methods, Marcel Dekker, New York, pp. 1–64.
Gee, G.W. and M.E. Dodson, 1981: Soil water content by microwave drying: A routine procedure. Soil Science Society of America Journal, 45, pp. 1234–1237.
11. Greacen, E.L., 1981: Soil Water Assessment by the Neutron Method. CSIRO, Australia, 140 pp.
12. Jackson, T.J. and T.J. Schmugge, 1989: Passive microwave remote sensing system for soil moisture: Some supporting research. IEEE Transactions on Geoscience and Remote Sensing, 27, pp. 225–235.
13. Magagi, R.D., Kerr, Y.H., 1997. Retrieval of soil moisture and vegetation characteristics by use of ERS-1 wind scatterometer over arid and semi-arid areas. Journal of Hydrology 188-189, 361–384.
14. Marthaler, H.P., W. Vogelsanger, F. Richard and J.P. Wierenga, 1983: A pressure transducer for field tensiometers. Soil Science Society of America Journal, 47, pp. 624–627.
15. Topp, G.C., J.L. Davis and A.P. Annan, 1980: Electromagnetic determination of soil water content: Measurement in coaxial transmission lines. Water Resources Research, 16, pp. 574-582.
16. Paul McMananmon, Field Guide to Lidar, SPIE Field Guides Vol FG36.
17. Keeler R. Norris, , Apparatus and method for reducing solar noise in imaging lidar, underwater communications and lidar bathymetry systems US Pat. 5192978
18. Thermal Infrared Characterization of Ground Targets and Backgrounds Sec. Ed, Pieter A. Jacobs, Tutorial Texts in Optical Engineering, SPIE Vol. TT70
19. Michael P. Finn, Mark (David) Lewis, David D. Bosch, Mario Giraldo, Kristina Yamamoto, Dana G. Sullivan, Russell Kincaid, Remote Sensing of Soil Moisture Using Airborne Hyperspectral Data, GIScience & Remote Sensing, 2011, 48, No. 4, p. 522–540
20. http://www.precisionhawk.com/ - precision agriculture services
21. http://www.seattletimes.com/business/agriculture-oriented-drone-firm-moving-to-seattle/
22. http://blogs.discovermagazine.com/drone360/2015/05/27/farmers-drones-robots-gps/#.VXSMOkZ8twE
23. http://www.techinvestingdaily.com/articles/agricultural-drone-investing/229
24. http://www.alltech.com/blog/posts/drones-and-potential-precision-agriculture
25. http://www.boeing.com/features/2013/07/corp-spuds-07-29-13.page
26. Buiteveld, H., J. H. M. Hakvoort, and M. Donze. 1994. The optical properties of pure water. Ocean Optics XII. SPIE 2258: 174–183.
27. Hale, G. M., and M. R. Querry. 1973. Optical constants of water in the 200nm to 200mm wavelength region. Applied Optics 12: 555–563.
28. Kou, L., D. Labrie, and P. Chylek. 1993. Refractive indices of water and ice in the 0.65mm to 2.5mm spectral range. Applied Optics 32: 3531–3540.
29. Palmer, K. F., and D. J. Williams. 1974. Optical properties of water in the near infrared. J. Opt. Soc. America 64: 1107–1110.
30. Smith, R. C., and K. S. Baker. 1981. Optical properties of the clearest natural waters (200–800nm). Applied Optics 20: 177–184.
31. R.A.Stermer, Y.Pomeranz and R.J. McGinty, U.S. Grain Marketing Research Center, Agricultural Research Service, U.S. Department of Agriculture, Manhattan KS., Cereal Chem. 54(2): 345-351, Infrared Reflectance Spectroscopy for Estimation of Moisture of Whole Grain., and references therein.
PART 3. RESONANT LASER TECHNOLOGY FOR ENVIRONMENTAL SENSING
A Laser Sensor for Selective and Precise Determination of Calcium and Strontium in Seawater from a Float Platform
INTRODUCTION
Lambdanetics manufacturers and markets light emitter, detector, and processing products for the research, industrial, and military markets The company currently focuses on developing IPSSL technology, a new and proprietary “atomic resonant” laser. IPSSL offers high performance in a compact package enabling laboratory-quality chemical analytical measurements in a light weight, autonomous platform. Applications include a sensor for the selective and precise measurement of calcium and strontium in seawater from a float platform, as presented and discussed below.
ACKNOWLEDGEMENT OF NOAA SUPPORT
Experimental work presented in this section was partially supported by the National Oceanic and Atmospheric Administration (NOAA) under contract number WC-133R-15-CN-0068. This support is gratefully acknowledged. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of NOAA.
OUTLINE
- The Problem of Ocean Acidification, De-Calcification, and the Human Food Chain
- Measurement Challenges
- The Innovation: New Sensor Offers Practical Solution to Analyzing Ocean Chemistry
- Sensor in Float Platform
- Product Development Plan
THE PROBLEM OF OCEAN ACIDIFICATION, DE-CALCIFICATION, AND THE HUMAN FOOD CHAIN
One of the major threats of climate change is the impact of ocean acidification on the human food chain. The uptake of anthropogenic atmospheric CO2 in the ocean is known to increase the acidity (lower the pH) due to the equilibrium formation of carbonic acid (H2CO3), and bicarbonate (HCO3-) and carbonate (CO32-) ions. It is estimated that the pH at the ocean surface may have decreased from 8.25 to 8.14 from 1751 to 1994 as a result, and the trend is currently continuing at a greater rate [1,2].
The human food chain is coupled to ocean acidification through the process of calcification. Calcification involves the precipitation of calcium carbonate (CaCO3), the major constituent of the shells of shellfish, skeletal structures, and coral reefs. Increased acidity tends to increase the saturation level of dissolved calcium ions (Ca2+) through the alteration of the CO32- concentration thereby decreasing the precipitation rate of CaCO3. Under extreme conditions calcification rates could actually go negative, which means that shells or coral would dissolve (or could not grow). Although calcium is the third most abundant species in the ocean, present at average levels of 10.3 mM/L (milli-moles per liter), only very small changes in Ca2+ concentration could have a large impact on calcification [2,3] rates. It is estimated that precision would need to be on the order of 5 µM/L (5 micro-moles per liter) to track the behavior of the oceans.
Strontium also plays an important role in seawater chemistry and calcification. For example, shell formation in certain gastropods has been shown to be sensitive to changes in Sr2+ concentration of only a few ppm under certain conditions. The interaction could be through the nucleation process of calcification sites. Under-nourishment of strontium at the larval stage can lead to the formation of deformed shells. The mechanically imbalanced structures can cause severely impair behavioral symptoms later in life. The strontium/calcium ratio is also related to biogeochemical cycling of calcium, and to ocean temperature. As the fifth most abundant species in seawater, present at about 0.091 mM/L, greater knowledge of the role of strontium is also needed [4,5,6,7,8,9,10,11,12].
MEASUREMENT CHALLENGES
Governments are charged with the stewardship of the environment by promoting policy for management, regulation, and restoration. Effective policy requires an understanding of the structure and behavior of the oceans, atmosphere, and related ecosystems. This understanding would ideally be based on the integration of research, analysis, and model development. At the core of research is sufficient data characterizing physical, chemical, and biological states of these systems.
Due to the sheer size of the earth, the cost and logistics of making high precision chemical measurements in either on-shore or specially equipped shipboard laboratories have been major obstacles. The use of remote and unattended platforms with integrated sensors and networked through global satellite systems is significantly reducing this cost by enabling on-site sample collection, sensing, and analysis. An example is the Argo system of over 3500 unanchored floats designed for remote salinity and pH determinations [13,14]. However, chemical analysis is much more sophisticated and challenging for several reasons. Although key chemical constituents are present at relatively high concentration, very small changes in concentration over time or from location to location need to be determined. In addition, the ocean contains many constituents that can interfere with one another during chemical analysis reducing measurement sensitivity. Most high precision analytical instrumentation requires special facilitization, including sources of high purity gases, high purity water, and ventilation. Consumable reagents and materials for standards are also necessary support. In addition, customized sample preparation is often required.
In consideration of developing an autonomous measurement sensor for calcium and strontium, several factors must be taken into account. These include selectivity, precision, reliability, dynamic range, and spatial and temporal variability. Sensitivity to environmental factors such as temperature, pressure, electromagnetic noise, and interfering chemical activity must also be taken into account. Power budget, consumables, and communication networking are also critical.
Some common analytical techniques have been broadly compared (see Table 1) on the basis of analytical performance and potential for autonomous operation. Titrimetric and voltammetric techniques are not considered due to special needs for sample preparation, reagent replenishment, and cell replacement.
Table 1. Comparison of projected performance of LFEFS to ISE, commercial flame photometry (FP), ICP-AES, and AAS for calcium (i).
| ISE (a) | Flame Photometry (b) | ICP-AES (c) | AAS | LFEFS | |
| Working Concentration | 0.5µM-1M | 60µM-5mM | 0.1µM-1mM | 0.1µM-1mM | 1µM-20mM |
| Precision (typical) | (e) | 10µM (0.1-5%) (l) | 5M (0.05%-1%) | 5µM (0.1-1%) | < 1µM (0.05%) |
| Optical Resolution | N/A | 5-10nm | 50pm | 5-50pm | 3-50pm |
| Shrinkability | Yes | Yes | No | No | Yes |
| Mechanicals | Can be handheld | 40lbs | 400lbs (Shimadzu) | 200lbs | 4-5" dia x 15-20" long |
| Facilitization (h) | No | Yes | Yes | Yes | No |
| Consumption | Yes | Yes | Yes | Yes | Yes |
“a”- PVC membrane; “b”- commercial system @422 nm; “c”- typical resolution @ 315 nm of 0.05 nm-
refs. [21,17,18,19]; ”d”- @461 nm; “e”- typical 10%, best 2% ref. 34; ”f”- Refs. [15,16,17]);
according to Ref. [25]; f- Refs.[ 21,17,18,19]; “g”- "typical" for conventional methods and projected for LFEFS;
“h”- compressed high purity gas tanks, gas for flame, etc.; “I”- data for strontium is similar to that for calcium.
Note: A is atomic mass equal to 40.08 for Ca and 88.62 for Sr; M=moles/liter; mM = M/1000; ppm = A x mM;
Ion selective electrode potentiometry (ISE) requires relatively simple instrumentation essentially consisting of a probe and a high impedance voltmeter. The technique utilizes ion exchange equilibrium between the analyzed solution and a solid inorganic or organic ion exchange membrane. The exchange of ions between the membrane and the solution creates an interfacial potential drop or increase, which can be measured against a suitable reference electrode. The interfacial potential change is logarithmically proportional to the ion concentration in solution. Unless specific chemical interactions are involved, the affinity of the membrane to various ions strongly depends on their ionic potential (i.e., the ratio of their charge to the radius). Due the similar ionic potentials and atomic radii of Ca2+ and Sr2+, the reaction of ion exchange membranes to these ions is non-specific. Electrodes are also generally highly susceptible to contamination by organic materials and heavy metals, and require frequent replacement.
One of the most common techniques for the determination of calcium and strontium is Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES). Analytes are fed into argon plasma (9500K), and the light intensity of the signature wavelength of each element is used to determine the concentration. The concentration of the analyte must often be adjusted by dilution or pre-conditioning with reagents to meet the working concentration window of the instrument. The size, weight, need for facilities support, and vulnerability to corrosion generally precludes ICP use in marine environments.
Of the techniques considered in this evaluation, flame photometry (FP) [4,15,16,17,18,19,20] perhaps has the greatest potential. FP is also an atomic emission spectroscopic technique [21] like ICP. Atomic excitation is generated by a flame (2000-2500K), but at a much lower temperature than ICP. Unlike ICP instruments, flame spectrophotometers on the market are generally limited to the analysis of Group I and II elements. The intensity of the resonant wavelengths of these elements is strong and easily resolved by inexpensive interference filters. The photoelectric signal is a direct function of the concentration of the particular element under test. Analytes may require some dilution to avoid self-absorption [9,15, 20].
Atomic Absorption Spectroscopy (AAS) relies on Beer-Lambert Law of light absorption. Absorbance of light is proportional to the concentration of atoms in their ground state. Precision and sensitivity for alkali or alkali earth metals are generally comparable ICP or FP. These instruments rely on hollow cathode lamps (HCL), which are known to emit multiple lines other than the primary resonance, and require filtering. In addition, these lamps cannot be electronically modulated, and if heterodyne signal detection is required, mechanical choppers are required. Such choppers have moving parts and are not amenable to design shrink. Finally, HCL tubes operate on high voltage. AAS instruments operate with graphite ovens [17] or flames, and require optical paths of at least 20-25mm to assure sufficient absorbance for detection. As a result, such designs are not readily shrinkable.
The ICP, AAS and FP all require repeated calibration using standard solutions.
The Innovation: New Sensor Offers Practical Solution to Analyzing Ocean Chemistry
A sensor is being developed based on IPSSL technology providing ICP-comparable precision, affordability, and compatibility with autonomous operation in a seaborne platform. This technique is referred to as Laser Fluorescence Enhanced Flame Spectrophotometry (LFEFS). LFEFS would generally be applicable to multiple components of seawater, such as calcium, strontium, barium, sodium, potassium, lithium, and mercury. Current activity is focused on calcium and strontium. A primary design objective is precision of 5 µM/L.
A schematic of the LFEFS sensor is shown in Fig.1. The IPSSL-Ca output beam probes the flame. A seawater sample is injected into a micro-flame by using a miniature and specially designed nebulizer. Calcium salts in the seawater are dissociated and atomized by the heat of the flame. These atoms resonate optically with the IPSSL-Ca beam creating blue laser induced fluorescence (LIF). This LIF is detected and electronically processed.
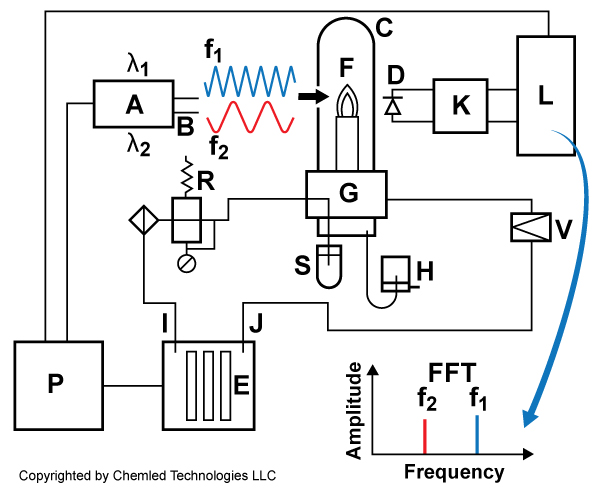
Figure 3-1. Schematic of LFEFS system showing integration of IPSSL-Ca-Sr with flame, nebulizer, and measurement electronics. “A” – IPSSL-Ca modulated at frequency f1, and IPSSL-Sr modulated at freq f2, “B” laser beams, “C” – burner chimney, “D” – Si photodetector set at 90 deg to the laser axis, ”K” -transimpedance amplifier, ”L”-digital signal processing with embedded FPGA –Fast Fourier Transform Xilinx IP-core, “R”- pressure safety relief valve, “G”-oxy-hydrogen, piezo nebulizer and aerosol mixing, ”S”- Valco sample feeder , ”H”-waste disposal, ”V”-control valve, ”P”-power supply, ”E”-electrolyzer, “I-”oxygen”, ”J”-hydrogen.
The laser drive current is directly modulated at a selected and fixed frequency in the kHz band allowing discrimination of the sample signal from the background noise and emissions from out-of-resonance, un-modulated species. Flicker (1/f) noise in the trans-impedance amplifier of the receiver is also reduced.
The LFEFS innovation is enabled by the incorporation of IPSSL technology. The advantages of employing IPSSL are as follows.
1. As an atomic resonant platform, a number of IPSSL devices are available to choose from to match the element to be analyzed. For example, IPSSL-Ca and IPSSL-Sr are perfect selections for the determination of calcium and strontium in seawater because the IPSSL laser line profile closely overlays the corresponding absorption profiles. (This overlay is discussed in Part 1 of the Technology and Markets section of this website.) The generation of LIF is highly element-selective. Combined with direct modulation techniques, good signal-to-noise and dynamic range are realized using conventional, inexpensive semiconductor detectors. ICP instrumentation, on the other hand, requires special, high resolution spectrographs, such as Echelle spectrographs, to resolve the rich spectrum in the high temperature plasma torch.
To illustrate the selectivity and stability of atomic resonant technology for this application, the output beam of an IPSSL-Ca device was passed through an oxy-hydrogen flame fed with a water-solution of calcium chloride. An unmistakable blue LIF (422.673 nm) was readily observed along the path of the laser, as shown in Figure 2.
2. The wavelength stability within the line profile ranges from 0.1 to 1 pico-meters over 12 hours depending on the particular device model. Such stability characteristics significantly outperform conventional, stabilized semiconductor laser. High stability promotes high analytical precision.
3. Operating optical power levels are sufficient to saturate the resonant transition, and quenching of exited atoms (i.e., through collisions with other constituents in the flame) is eliminated. Such conditions enhance precision and signal-to-noise of the measurement.
4. LFEFS operates on atomic transitions terminated in ground states, as does AAS, whereas ICP detects excited states. Because the concentration of ground states exceeds that of excited states (governed by Boltzmann distribution), detected signals are higher in LFEFS offering better precision and signal-to-noise performance. This is particularly true for group I and II elements. Compared to AAS, LFEFS is amenable to design shrink. The optical beam can also be chopped electronically for better signal-to-noise.
5. IPSSL is “perpetually” calibrated by virtue of EAS offering relative design simplicity, and reduced cost of hardware, operation, and maintenance.

SENSOR in FLOAT PLATFORM
The LFEFS sensor is being designed as a package for autonomous float or platform applications. Acquired data would be communicated through an appropriate network for storage and analysis. Electrical power could be solar- or wind-harvested. Fuel for the flame would be hydrogen and oxygen produced by the electrolysis of seawater eliminating the need for fuel storage and replenishment. An internal flame temperature estimated at about 2600°C would be sufficient to de-solvate and atomize the sample, and to oxidize organic constituents.
The basis of the LFEFS chemometrics is the widely known “standard addition” method (21, 20, 24), and consumable standard solutions will have to be stored on board. The design objective is to reduce the injection volume per calibration to as little as 1 µl, which would be sufficient for several years of operation. A primary design objective is precision of 5 µM/L.
REFERENCES
1. Hennessy, K., B. Fitzharris, B.C. Bates, N. Harvey, S.M. Howden, L. Hughes, J. Salinger, and R. Warrick. 2007. Australia and New Zealand. In Climate change 2007: Impacts, adaptation and vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Edited by M.L. Parry, O.F. Canziani, J.P. Palutikof, P.J. van der Linden, and C.E. Hanson. Cambridge University Press, pp. 507-540.
2. Feely, R. A., C. L. Sabine, K. Lee, W. Berelson, J. Kleypas, V. J. Fabry, and F. J. Millero. 2004. Impact of Anthropogenic CO2 on the CaCO3 System in the Oceans. Science 305:362-3663.
3. Riebesell, U., I. Zondervan, B. Rost, P. D. Tortell, R. E. Zeebe, and F. M. M. Morel. 2000. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 407:364-367.
4. Karin E.Limburg, The Biogeochemistry of Strontium: a review of H.T. Odum’s contributions, Ecological Modeling 178 (2004) 31–33.
5. Gerald Langer, Calcification of selected coccolithophore species: strontium partitioning, calcium isotope fractionation and dependence on seawater carbonate chemistry. – Dissertation, 2005, Univ. of Bremen.
6. H.Tabouret, et. al., Simultaneous use of strontium:calcium and barium:calcium ratios… Marine Environmental Research July 2010, Volume 70, Issue 1, Pages 35-45.
7.Gao, K., Y. Aruga, K. Asada, T. Ishihara, T. Akano, and M. Kiyohara. 1993. Calcification in the articulated coralline alga Corallina pilulifera, with special reference to the effect of elevated CO2 concentration. Marine Biology 117:129-132.
8.Lea, D. W., T. A. Mashiotta, and H. J. Spero. 1999. Controls on magnesium and strontium uptake in planktonic foraminifera determined by live culturing. Geochimica et Cosmochimica Acta 63:2369-2379.
9.Marubini, F., and M. J. Atkinson. 1999. Effects of lowered pH and elevated nitrate on coral calcification. Marine Ecology Progress Series 188:117-121.
10.Rickaby, R. E. M., D. P. Schrag, I. Zondervan, and U. Riebesell. 2002. Growth rate dependence of Sr incorporation during calcification of Emiliania huxleyi. Global Biochemical Cycles 16:1006,10.1029/2001GB00140.
11. Travis S.Elsdon and Bronwyn M.Gillanders, Strontium incorporation into calcified structures…,Marine Ecology Progress Series, Vol 285 233-245,2005.
12. J.Guelinckx et. al., Relating otoliths to water [Sr/Ca] ratios, Journal of Experimental Biology and Ecology www.vliz.be/imisdocs/publications/133959.pdf.
14.Oceanography, V.25, No.1, March, 2012.
15. Tsaihwa J.Chow and Thomas G. Thompson, Analytical Chemistry, 1955, 27(6), pp 910-913.
16. T.J.Chow and T.G.Thompson, Flame Photometric Determination of Strontium in Seawater, Anal.Chem., 1955,27(1),18-21.
17. Manual BWB Technology, UK, http://www.somatco.com/BWB-1_D66-18.pdf, flame photometry Ca limits of detection 1.0 ppm, optimal range above 2.5 ppm.
18. Perkin Elmer, http://www.perkinelmer.com/CMSResources/Images/44-74318APP_TraceMetalsinDrinkingWaterbyOptima7000.pdf, Ca detection limit 0.5 ug/L – i.e.0.5 ppb (ICP OES).
19. Metal Vapours in Flames, by C.Th.Alkemande, Yj.Hollander, W.Snellman, and P.J.Th.Zeegers, Pergamon Press,ISBN 9781483285375.
20. J.K. Fawcett and J.C. Pathol,1961 Sep;14:463-9.
21. SOP For the Determination of Calcium, Magnesium, Sodium, Potassium by Inductively Coupled Plasma-Optical Emission Spectroscopy, SOP Number AN-0016.6 rev 6.0 December 1, 2009. National Atmospheric Deposition Program Central Analytical Laboratory.
22. R. Sobczynski et al, "Diode Arrays May Light up Compact Spectrometers", Laser Focus World 1995-Vol. 30, pp. 75-8 1.
23. R.Sobczynski, W.G.Fateley,US Patent 05488474 Hadamard phasing in multisource array.
24.A.K.Jain, V.K.Gupta,J.R.Raisoni, Strontium(II) selective potentiometric sensor…, Sensors, 2004,4,115-124.
